How Many Water Molecules Are in 5.2 Moles of Water
1 mole 6022 1023 molecules So number of moles present in 57 1023 molecules of water 57 1023 6022 1023 0946 moles Hope this helps. How many water molecules are in 52 moles of water.

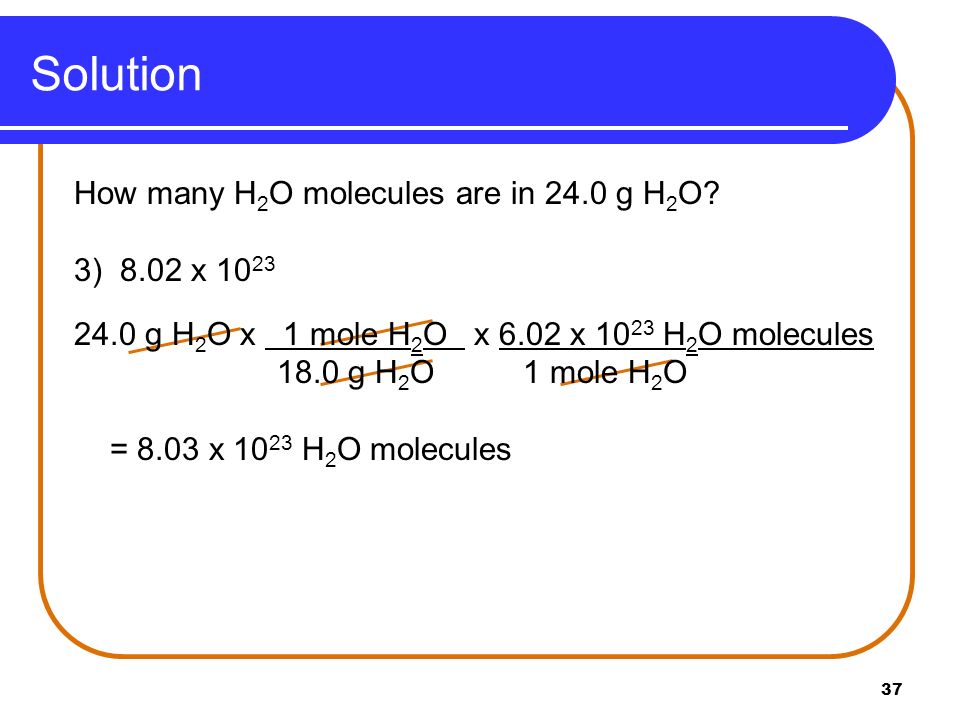
7 5 Molar Mass And Calculations Ppt Download
How many chlorine molecules are in 65 moles of chlorine.
. From the periodic table we see the atomic weight of hydrogen is 10079 and the atomic weight of oxygen is 159994. We assume you are converting between molecule and mole. 8638 x 10 -24.
For every 1 mol there are 602 1023 molecules. This means 1 mole of hydrogen weighs 10079 grams and 1 mole of oxygen weighs. Therefore for 5 moles of water there is 5 x 6022x1023 3011x1024 molecules of water.
For each mole of anything there is 6022x1023 molecules. How many moles of sulfur dioxide are in 226 x 1033 sulfur dioxide molecules. A mole of water molecules would be 2 moles of hydrogen atoms plus 1 mole of oxygen atoms.
31 x 10 24. 602 x 10 23. And 555093 moles TIMES 60221 x 1023 atoms per mole.
How many water molecules are in 52 moles of water. You can calculate the number of molecules from moles by using Avogadros constant 602 1023 moleculesmol. The answer is 60221415E23.
How many sulfur dioxide molecules are in 28 moles of sulfur dioxide. The _____ bonds between water molecules lead to waters cohesion. How many water molecules are in 52 moles of water H2O.
24 x 10 23 molecules. 25 mol 602 1023 1 mol 15 1024 molecules. 31304 x 1024 C.
Note that rounding errors may occur so always check the results. 602 x 10 23. Atomic mass is the number of grams per mole of the element.
15 x 10 24 molecules. Molecules or moles The SI base unit for amount of substance is the mole. When the concentration of molecules on both sides of a membrane is the same the molecules will.
31304 x 10 24. How many molecules in 1 moles. How many water molecules are in 52 moles of water.
8638 x 10-24 - 24570002. To produce 12 moles of water how many moles of oxygen gas are needed. 52 x 1024 D.
And 1000 grams water DIVIDED BY 18015 grams per mole EQUALS 555093 moles. How many water molecules are in 40 moles of water. You can view more details on each measurement unit.
42 x 10-24 molecules. How many moles of O 2 are their in 896 liters. 1 molecules is equal to 1660538863127E-24 mole.
602 x 1023 B. Number of moles massmolecular mass 2518 0138 moles 1 mole is 602 x1023 molecules 0138 x 602 x 1023 836 x 1022 molecules. A 26 long painting is how many yards long.
How many water molecules are in 25 moles of water.

8 1 Reacts With 3 Mol Of O2 Produces 1 Mol Of Al2o3 Ppt Video Online Download

How Many Water Molecules Are In 5 2 Moles Of Water Brainly In
No comments for "How Many Water Molecules Are in 5.2 Moles of Water"
Post a Comment